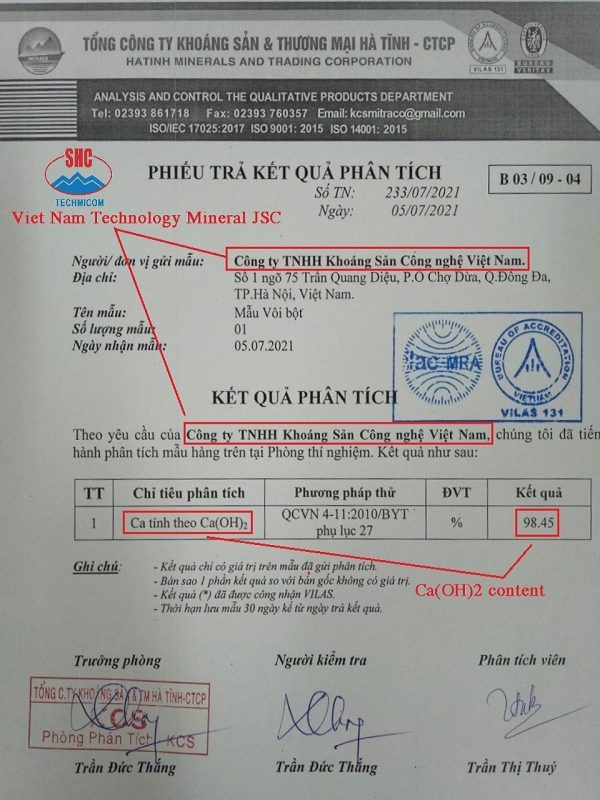
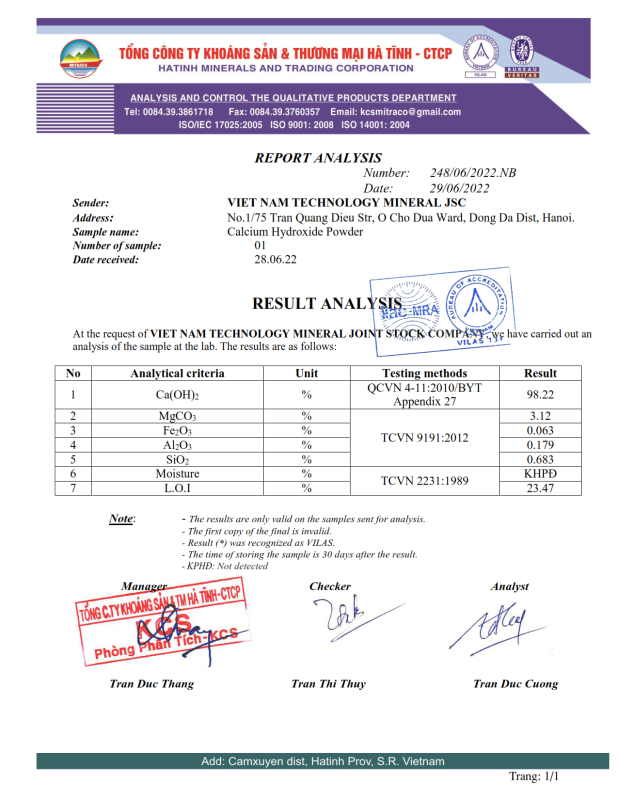
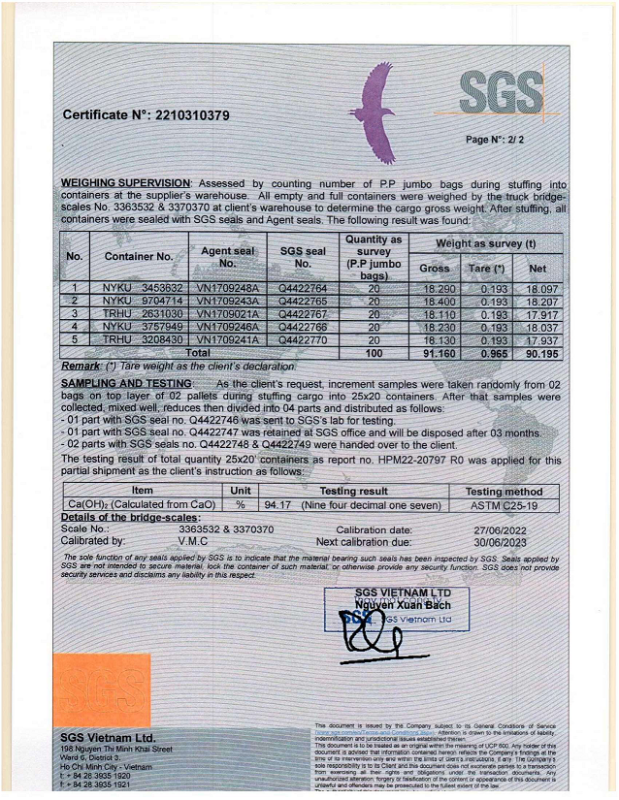
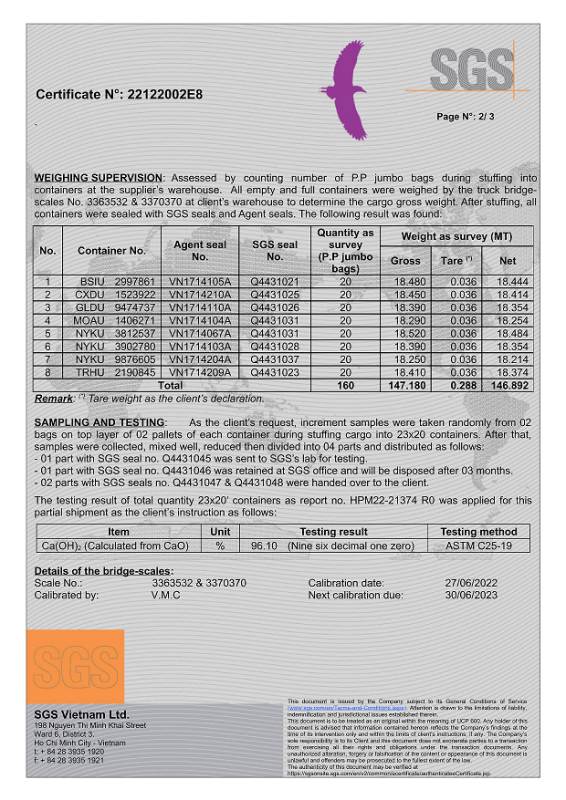
No products in the cart.
HYDRATED LIME CALCIUM HYDROXIDE
SKU: N/A
Price : Contact
Hydrated Lime Calcium Hydroxide Powder, commonly known as slaked lime, is an alkaline product made by mixing quicklime with water. It is used mainly in construction, water treatment, and agriculture, it helps neutralize acids in soil, treats wastewater, improves the strength of cement and mortar, removes toxins from flue gases, and aids in tanning hides. As a versatile industrial chemical, calcium hydroxide has the ability to adjust pH levels, react with acids, and precipitate unwanted compounds from solutions.