No products in the cart.
QUICKLIME LUMP
SKU: N/A
Price : Contact
Quicklime, also known as calcium oxide, is a highly caustic alkaline substance produced by calcining limestone at high temperatures. It is commonly used in construction materials, steel production, wastewater treatment, and chemical processes due to its ability to react violently with water and neutralize acids. Quicklime has a white powder, lump or granular appearance and requires proper protective equipment when handling due to its corrosive properties. Its production from limestone makes it a widely used industrial chemical worldwide.
- Other name: Calcium Oxide, Burnt Lime, Calcined Lime, Unslaked Lime
- Chemical Formula: CaO
- Manufacturer: Vietnam Technology Mineral JSC
- Quicklime is created by heating limestone (which is calcium carbonate or CaCO3) to high temperatures in a kiln, causing it to undergo calcination
- The chemical reaction during calcination can be written as CaCO3 → CaO + CO2
- When water is added or mixed into quicklime, it undergoes hydration and develops intense heat. This reaction is called slaking of lime
- It has a very high alkaline pH of around 12.5, making it strongly caustic and able to damage skin, eyes, and respiratory tract on contact. Proper precautions must be taken when handling it
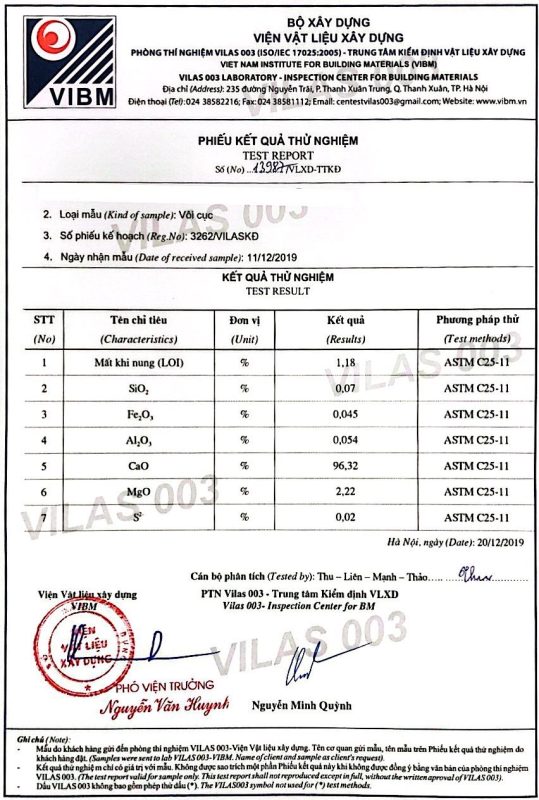
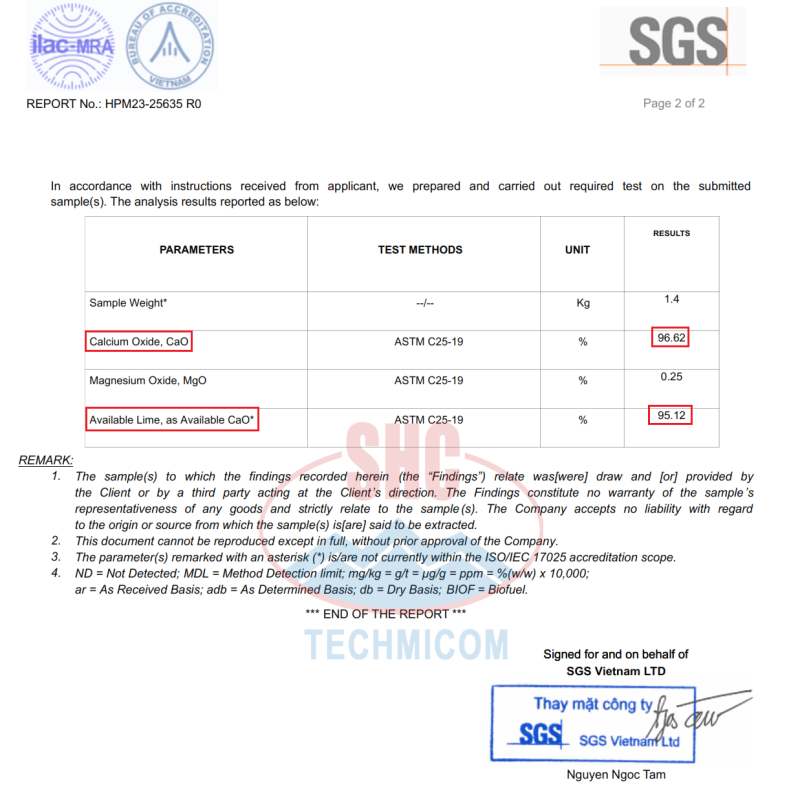
- Calcium oxide (CaO) content – The highest quality quicklime will be at least 90% pure calcium oxide
- Particle size – Finer particles (<1mm) offer a larger surface area and faster reactivity
- Moisture content – Quicklime picks up moisture easily which decreases reactivity. High-quality lime will have less than 1-2% moisture content
- Chemical impurities – Minerals like magnesia (MgO) are common but desirably under 2.5%. Trace heavy metals like lead or arsenic should be virtually nonexistent
- Consistency – Well-burnt, high-density quicklime will be uniform in particle size, density, and reactivity across batches
- Reactivity – Faster, more exothermic reactions when slaked indicate purer, livelier lime that mixes/sets quicker. 24 hours is ideal setting time
- Storage stability – Long shelf life with no detectable decline in properties over 6-12 months stored properly demonstrates consistent burning
- Source traceability – Knowing limestone source and kiln conditions results in more predictable, reliable quicklime quality batch to batch
- Proper burning is key – Quicklime from modern rotary kilns tends to offer the best, most consistent quality versus older fixed-grate kilns

Specification
</tr>
&amp;lt;table style=”border-collapse: collapse; width: 100%; height: 147px;”>
ITEMSRESULT</span></td>ITEMSRESULT</span>=”color: #282828; font-size: 16px;”>CaO≥92%S≤0.08%</span>MgO≤1.5%L.O.I<span style=”color: #282828; font-size: 16px;”>≤4.0%</span></tr>Fe2O3≤0.05%Slaking Time04 Minutes</span><span style=”color: #282828; font-size: 16px;”>Al2O3≤0.5%tyle=”color: #282828; font-size: 16px;”>Slaking Degree90ºCSiO2<0.68%Size10-80 mmReactivity<span style=”color: #282828; font-size: 16px;”>≥300 mlColorMilky White
Packaging and Loading
&amp;lt;table style=”border-collapse: collapse; width: 100%; height: 144px;”>
Bag sizeSmall Bag 40kg | Jumbo Bag 1.4MTBag materialPP + PE + Nylon + UV resistanceAccessoriesPallet + Wrapping Film + Strapping BeltLoadingUp to 28MT/FCL 20ftCustomized bag printing>100 metric tons: Free
<100 metric tons: 150 USD/color
Application of Quicklime Calcium Oxide Lump
<td style=”width: 15.8691%; height: 31px; text-align: center;”>Flue gas desulfurization
<table style=”border-collapse: collapse; width: 100%; height: 51px;”>
style=”width: 19.5708%; height: 31px; text-align: center;”&gt;Water treatment/Wastewater treatment</span>Metallurgy Paper productionSoil treatment</span><span style=”color: #282828; font-size: 16px;”>Construction <span style=”color: #282828; font-size: 16px;”>Chemical manufacturingAgricultureMiningtyle=”color: #282828; font-size: 16px;”>Sugar refiningle=”color: #282828; font-size: 16px;”>Swimming poolsRoad constructionGlassmaking TanningPetroleum refining
related products
( SHC GROUP is the REPUTED BRAND in mineral field from Vietnam )





















